DYKE'S INSTRUCTION No. 88 (Supplement)
Fig. 6. Large view shows action of "over-lap" valve timing
in permitting exhaust gas to flow across cylinder into in-take
manifold, when intake and exhaust valves are open at same time,
at beginning of intake stroke: ENGINE under L I G H T LOAD, NEARLY
CLOSED THROTTLE.
Small view at right shows re
sulting proportions of air, gasoline vapor, and exhaust at beginning
of compression. Note large quantity of exhaust.
With this valve timing, it is usually more difficult to get smooth
engine operation at idling and low speed.
It is particularly necessary that the valve tappet clearance be
kept uniform, as varying degrees of overlap in different cylinders
will give them different strengths of charge, making irregular
firing which cannot be cured by any carburetor adjustment.
Misfiring on the comeback or when coasting: The least amount of
air in cylinder, and the most unfavorable conditions for firing
are obtained when the engine is turning over at high speed with
the throttle closed to the idling position, when each cylinder
has time to receive only a very small air and fuel charge.
This condition is reached when the car is coasting down a steep
hill with gears in mesh and the throttle fully closed. Such a condition
also exists temporarily when the engine is raced from idle up to
high speed and the throttle quickly closed.
Owing to the small amount of air and very high percentage of exhaust
dilution, the burning in the cylinder is very slow, while on account
of the high engine speed, there is very little time for each combustion
to be completed; so that with small defects such as intake leaks,
exhaust leaks or weak ignition, the engine is very apt to miss
or fire in the muffler under conditions just described. The tendency
to misfire is less with full advanced spark.
Vaporization
Vaporization of fuel necessary: It should be understood that gasoline
in liquid form or drops will not burn efficiently in an engine.
In nearly all forms of burning or combustion with which we are
acquainted, complete burning must be preceded by vaporization.
Under the flame of a candle or kerosene lamp, there is a heated
region filled by vapor of the tallow, paraffine or kerosene, the
wick serving as a means for graduating the supply and controlling
the formation of this vapor.
Wood and coal commonly burn by the process of distillation or evaporation
into inflammable gas before burning, and so on; and if we attempt
to burn any of these common inflammable substances, including gasoline
itself, by heating them highly when they are not intimately mixed
with air, we obtain smoke, soot and carbon or coke deposit.
Parts of the fuel charge that can be used. In the automobile engine,
with the short time allowed for explosion, only vaporized fuel
can burn and it is, therefore, necessary in any work with mixture
pro-portion that the extent. of fuel vaporization be taken into
account.
At 32° F. At 90° F. At 130° F.
Fig. 8. Light section shows proportion of full liquid charge of
average motor gasoline that can evaporate at temperatures specified.
Illustration (Fig. 8) shows the percentages of our present average
of gasoline which will evaporate in air at 32° F. temperature,
at 90° F., a temperature comfortably lukewarm to the hand,
and at 130° F., a temperature about as hot as the hand can
stand.
It will be noted that the proportion of fuel charge which will
evaporate is greatly different at these different temperatures,
the reason being, of course, that gasoline is really made of a
number of different ingredients, some of which are very volatile
and others of which are much harder to evaporate than water.
When the engine is cold, only a part of the fuel will vaporize
in the cylinder and the amount of fuel fed into the cylinder must
be so great that there will be enough of the small proportion vaporizing
to give a complete vapor charge for burning with the air.
Illustration (Fig. 9) shows the actual size of a charge of average
present-day motor gasoline which must be fed to a 3%"x4%" cylinder
to give normal combustion at zero degrees Fahrenheit cylinder temperature,
compared with the amount necessary after the cylinder has become
warm on the inside from being fired one hundred or more times.
Fig. 9. Actual size of liquid gasoline charge that must be fed
to one cylinder of 3engine, for best power, at temperatures specified.
Under normal operation
The part of the fuel which does not vaporize Is, of course, heated
very highly during the explosion, with the result that it will
largely change to carbon and coke, part of which is deposited on
the cylinder walls and part carried out with the exhaust.
Vaporization in the carburetor and intake systems: The foregoing
has dealt only with the conditions in the engine cylinder.
Fig. 7. Engine turning over at high speed with throttle closed
to low idling position. Note that air and fuel vapor charges are
much smaller than with normal idling as in Fig. 3.
in cold cylinder at 0° F.
Previous page 1927
Supplement Home Next page 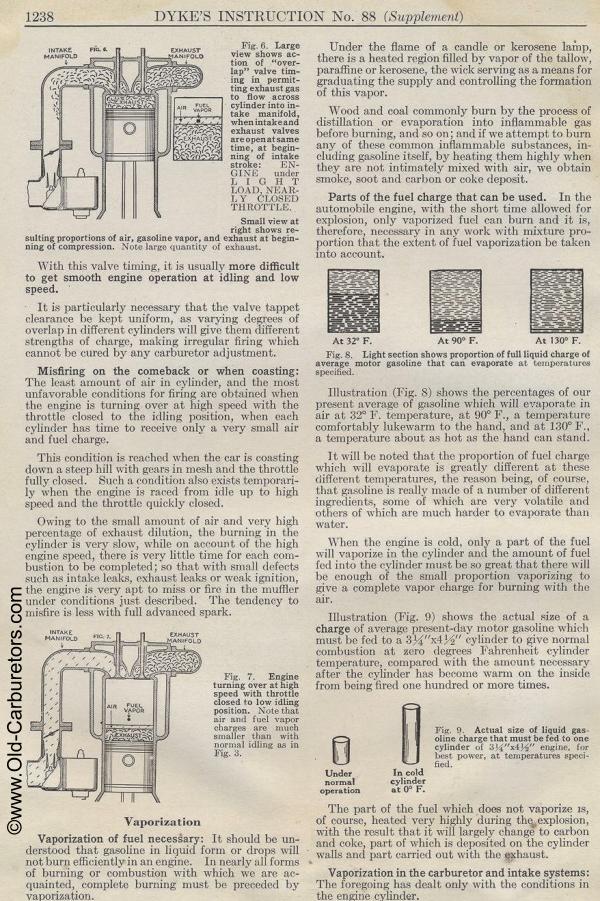
|
